Advance Your Skills with Expert-Led Training
Join our Pharmacovigilance Course and Gain the Expertise Needed to Advance in one of the Fastest-Growing Sector in Bangalore.
Our Features :
 100% Placement Assistance
100% Placement Assistance 27+ Yrs Exp. Trainers
27+ Yrs Exp. Trainers Best & Affordable Price
Best & Affordable Price Interview Preparation
Interview Preparation Ratings
4.9/5
Ratings
4.9/5
Demo Lecture For Online ! Hurry Up
2900+
Students
Flexible
Batch Timing
3 Months
Duration
Online
Teaching
100%
Placement
11 Nov.
11 Nov.
Upcoming Batch
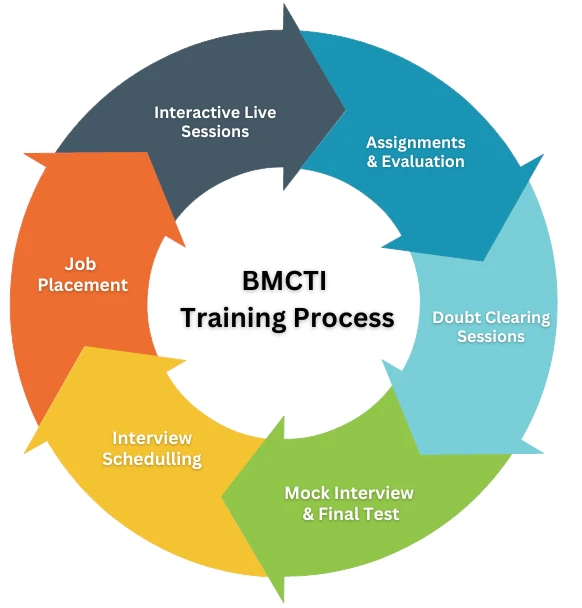
BMCTI offers the best Pharmacovigilance course in Bangalore with 100% placement assistance. We cover all the important aspects of pharmacovigilance includes Clinical Research, Drug Development, Introduction to (GCP)-ICH E6, PV, and Safety Reporting. Trainers having 27+ years of understanding works at BMCTI to train candidates. With a passion of building a strong healthcare system in India, BMCTI has trained more than 3000 candidates.
Register to confirm your seat. Limited seats are available.
Pharmacovigilance is like a drug investigator aiming at identifying and responding to the drug safety. Ultimately, this branch of clinical research deals with identification of the hazardous pharmaceutical products and minimizing the risk of harm. They are the bridge of trust between the drugs and the patients. The pharmacovigilance is responsible for performing following duties:
 Monitor newly released drugs
Monitor newly released drugs Evaluate Data
Evaluate Data Ensure the medicines are effective and safe for every kind of patient
Ensure the medicines are effective and safe for every kind of patient Reject false safety signals
Reject false safety signals Share information with the regulatory agencies
Share information with the regulatory agencies Creating and implementing risk management plans
Creating and implementing risk management plans Cognizant
Cognizant Ranbaxy
Ranbaxy Pfizer
Pfizer Novartis
Novartis Matrix laboratories ltd.
Matrix laboratories ltd. Dr. Reddy’s lab & etc.
Dr. Reddy’s lab & etc.Pharmacovigilance is a shield of protection to public health. To bring the medicine in market, it is necessary to ensure safety and effectiveness. Pharmacovigilance team is the detective assessing risks, making connections, and taking appropriate action on the front line. Candidates according to their skillsets and talent can choose their career in any of the below mentioned fields of pharmacovigilance.
 Drug Safety Associate
Drug Safety Associate Drug Safety Scientist
Drug Safety Scientist Validation
Validation Aggregate Report Scientist
Aggregate Report Scientist Regulatory Associate
Regulatory AssociateWhile experience, certification, location, and other factors like skills & knowledge can influence salary range, here's a general range of what Clinical Research professionals in India can expect to earn annually:
Some of the major job roles and average annual salaries that one can prefer are:
| Entry-level: | Rs.1.8 to 4.5 lakh |
| Experienced: | Rs.5.5 to 8 lakh |
| Quality Manager: | Rs.7 to 9 lakh |
| Drug Safety Coordinator: | Rs.7.5 to 8 lakh |
| Senior Research Scientist: | Rs.6 to Rs.9.5 lakh |
| Clinical Research Coordinator (CRC): | ₹5 - ₹8 lakh |

BMCTI has a thorough curriculum tailored particularly to prepare the candidates according the industry standards and regulations of pharmacovigilance. BMCTI provide training in technical skills like the use of Pharmacovigilance Databases and Software (Oracle Argus). These tools help in tracking ADRs, generating safety reports, and keeping up with regulatory requirements. Our e-learning platform provided you with daily live sessions, access to quality study material, and flexible session timings for candidates who are working professionals. For enhancing your skills in pharmacovigilance practices, we design 5-6 mock interviews, the mocks will help you understand the patterns of interview process and face challenging questions easily. After successful completion of course and training, BMCTI award the candidates with Pharmacovigilance Certification. Clinical research industries highly recommend our certified candidates for extensive job opportunities. If you are planning a career in Pharmacovigilance, then BMCTI is a key to your dream job!
B Pharmacy
B.Sc., M.Sc.
Nursing
Any life science discipline

3 Months

Onetime payment: 20,000/-
Installments: 30,000/-
BMCTI trainers have been involved in the Pharmacovigilance Courses for more than 27 years. Our trainers are Life Science Professional Graduates and Post Graduate. After completion of their graduation, they have worked with different healthcare Companies. Their dedication to contribute to your success is certain. Learn from the best educators of the BMCTI training institute to become a Pharmacovigilance professional.

 Introduction to Clinical
Research
Introduction to Clinical
Research Terminologies in Clinical
Research
Terminologies in Clinical
Research Advantages of CR in India
Advantages of CR in India
 Overview of Drug Development
Overview of Drug Development
 Clinical trial phases
Clinical trial phases BA/BE Studies
BA/BE Studies Drug Development Notes
Drug Development Notes Drug Development Quiz
Drug Development Quiz History Development of
International Regulations in Clinical
Research
History Development of
International Regulations in Clinical
Research
 Good Clinical Practices
Good Clinical Practices Ethics committee
Ethics committee Investigator Responsibilities
Investigator Responsibilities
 Sponsor Responsibilities
Sponsor Responsibilities Protocol and Investigator
Brochure
Protocol and Investigator
Brochure Essential Documents
Essential Documents Adverse Event Reporting
Adverse Event Reporting
 Recording of Event
Recording of Event Medical Management of
Adverse Events
Medical Management of
Adverse Events Handling Death
Handling Death Unbinding of Drug
Unbinding of Drug Clinical Safety and
Pharmacovigilance
Clinical Safety and
Pharmacovigilance E2A- Clinical Safety and
Data Management
E2A- Clinical Safety and
Data Management Media 436
Media 436 Naranjo Assessment
Naranjo Assessment Order and Formulate to
Determine the Quantum
Order and Formulate to
Determine the Quantum SAE Reporting
SAE Reporting Overview of PV
Overview of PV
 History of PV
History of PV ADR Classifications
ADR Classifications AE_SAE Reporting Process
AE_SAE Reporting Process Introduction to Aggregate Reporting
Introduction to Aggregate Reporting CDM- 21 CFR Part 11
CDM- 21 CFR Part 11 CRF_Design_2
CRF_Design_2 Introduction to CDM
Introduction to CDM Data Entry Methods
Data Entry Methods Query Management
Query Management Source data validation
Source data validation
 Future of Data Management
Future of Data Management
 Clinical Trial Start Up
Clinical Trial Start Up
 Clinical Trial Conduct
Clinical Trial Conduct
 Clinical Trial Close Out
Clinical Trial Close Out
 100% Placement Assistance
100% Placement Assistance  Unlimited Placement Calls
Unlimited Placement Calls 15+ Years Industry Experts
15+ Years Industry Experts  Mock Interviews
Mock Interviews Affordable Fees
Affordable Fees Interactive Live Sessions
Interactive Live Sessions Pay in Installments Options
Pay in Installments Options Career Guidance
Career Guidance Resume Preparation
Resume Preparation  Individual Mock Tests
Individual Mock TestsCandidate can easily apply through our website for Pharmacovigilance Courses with 100% placement assistance. Admission officers will review your application and contact you for further process.
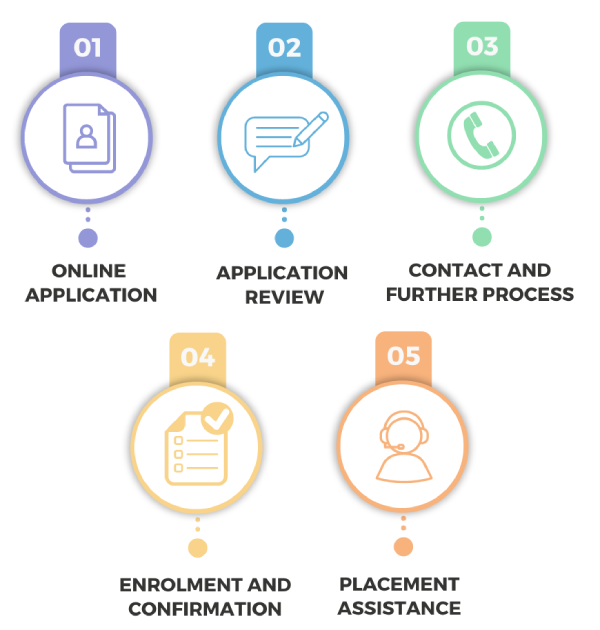
The objective of the course is to skill candidates and make them job ready with good salary packages. The course talks about the benefits of earning a pharmacovigilance certification for candidates thinking to excel in regulatory affairs. After completing the required expertise in the pharmacovigilance course at BMCTI, the candidates are awarded with a certificate. By certifying the trained-candidates, we create a standard of excellence. More than 2500+ students trust BMCTI. We give candidates the support needed to gain employment opportunities in pharmacovigilance. Obtaining a Certified Pharmacovigilance Professional (CPVP) certification demonstrates your expertise. BMCTI certified candidates are desired highly by the top healthcare and clinical research industries.














"The medical coding course at BMCTI was a game-changer for me! The trainers were highly skilled and passionate about their subject. Thanks to the institute's excellent support, I secured a placement in a reputable organization."
"Thank BMCTI for your proper guidance for clinical research course. Specially thanks to my mentors for proper guidance not only for syllabus but for interview preparation she helps me lot. I learn here complete clinical research in that they covered PV, CR, RA, MW ICH-GCP & ETC."
"Respected Mam,I am immensely grateful for your unwavering support throughout my medical coding journey. Your guidance and encouragement were instrumental in securing this job. Thank you for believing in me."
"I had a wonderful experience learning medical coding. The instructor created a friendly and supportive learning environment. The course was well-organized and made complex topics understandable."
"I wanted to do a carrier in Clinical Research and one of my friend suggest me to visit BMCTI. The institute provides an exceptional platform for aspiring professionals. I strongly recommend it to anyone seeking a rewarding career in clinical research."
"I found the clinical research course to be incredibly informative. The instructors were excellent at explaining complex topics, and I appreciate the institute's commitment to student placement."
Pharmacovigilance is responsible for inspecting newly released drugs and evaluating data. Further responsibilities include rejecting false safety signals and sharing information with regulatory agencies.
Steps to be a pharmacovigilance professional:
The average salary for pharmacovigilance professionals is Rs.2.8 lakh per annum to 5.5 lakh per annum.
Yes, we provide 100% placement assistance through unlimited interview calls.